ńĀöń®ČĶ½¢µ¢ć
A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging
MSC
Author
Master
Date
2015-08-12 16:47
Views
1019
A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging
Werner syndrome (WS) is a premature aging disorder caused by WRN protein deficiency. Here, we report on the generation of a human WS model in human embryonic stem cells (ESCs). Differentiation of WRN-null ESCs to mesenchymal stem cells (MSCs) recapitulates features of premature cellular aging, a global loss of H3K9me3, and
changes in heterochromatin architecture. We show that WRN associates with heterochromatin proteins SUV39H1 and HP1a and nuclear laminaŌĆōheterochromatin anchoring protein LAP2b. Targeted knock-in of catalytically inactive SUV39H1 in wild-type MSCs recapitulates accelerated cellular senescence, resembling WRN-deficient MSCs.
Moreover, decrease in WRN and heterochromatin marks are detected in MSCs from older individuals. Our observations uncover a role for WRN in maintaining heterochromatin stability and highlight heterochromatin disorganization as a potential determinant of human aging.
Werner syndrome (WS), also known as adult progeria, recapitulates certain aspects of human physiological aging. WS is caused by mutations in the WRN gene, resulting in loss of WRN expression or function. WRN protein plays roles in DNA replication, transcription, repair, and recombination, as well as telomere maintenance,
indicating that one of the major causes for WS pathogenesis relates to genomic instability. Epigenetic alterations have been associated with cellular aging in diverse model organisms. In humans, somatic cells derived from patients with premature aging syndromes are characterized by loss of heterochromatin marks. However, it is unclear whether epigenetic dysregulation is involved in WS pathogenesis.
Generation of patient-specific induced pluripotent stem cells (iPSCs) represents a promising avenue to model and study human aging and aging-associated disorders. WS-specific iPSC lines may constitute an ideal source for in vitro modeling of WS. However, we found that WS patient fibroblast lines deposited in different cell banks presented severe karyotypic abnormalities and secondary DNA mutations associated with advanced stages of WS pathology. To create an unbiased human WS cellular model, we sought to generate an isogenic WS embryonic stem cell
(ESC) line by knocking out exons 15 and 16 of the WRN gene encoding the conserved DNA helicase domain. After two rounds of homologous recombination using helper-dependent adenoviral vector (HDAdV), we successfully generated homozygous WRN-null ESC lines (ESCs-WRNŌłÆ/ŌłÆ ). ESCs-WRNŌłÆ/ŌłÆ expressed pluripotency markers, maintained normal karyotype, and could differentiate into all three germ layers.
ESCs-WRNŌłÆ/ŌłÆ lacked detectable WRN protein, as determined by Western blot using antibodies specific to the N or C terminus of WRN. No difference in cell cycle kinetics and cell growth rate between wild-type and WRN-null ESCs was observed.
WS patients are mainly characterized by premature aging pathologies associated with degeneration of mesodermal tissues, i.e., osteoporosis, atherosclerosis, and gray hair. We hypothesized that WS patients may suffer from an accelerated exhaustion of the mesenchymal stem cell (MSC) pool. This was tested by differentiating ESCsWRNŌłÆ/ŌłÆ intoMSCs. MSCs-WRNŌłÆ/ŌłÆ expressedMSCspecific cell surface markers CD73, CD90, CD105; lacked expression of MSC-irrelevant antigens, including CD45, CD34, and CD43 (fig. S3A); and could differentiate into osteoblasts, chondrocytes, and adipocytes. Upon serial passaging, WRN-deficient MSCs recapitulated major phenotypes of premature aging, including premature loss of proliferative potential, increased number of senescence-associatedŌĆōbgalactosidase (SA-b-gal)ŌĆōpositive cells, up-regulated expression of aging-associated genes p16Ink4a and p21Waf1, and activation of senescence-associated secretory phenotype (SASP) Moreover, when WRN-deficient MSCs expressing luciferase were transplanted into the muscle of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, they underwent an accelerated attrition compared to wild-type MSCs. These results demonstrated that the loss of WRN promotes premature senescence in MSCs.
WRN deficiency in MSCs resulted in elevated DNA damage response (DDR), indicated by increased nuclear foci for 53BP1, g-H2AX, and phosphorylated ATM/ATR substrates. Restoration ofWRN activity by lentivirus-mediated expression in MSCs-WRNŌłÆ/ŌłÆ resulted in partial alleviation of DDR and cellular senescence (fig. S4,
D and E). To investigate potential chromosomal abnormalities resulting from the loss of WRN protein,
we performed genome-wide copy number variation (CNV) analysis by deep sequencing. In the time frame examined, genomic integrity was minimally affected in MSCs-WRNŌłÆ/ŌłÆ.
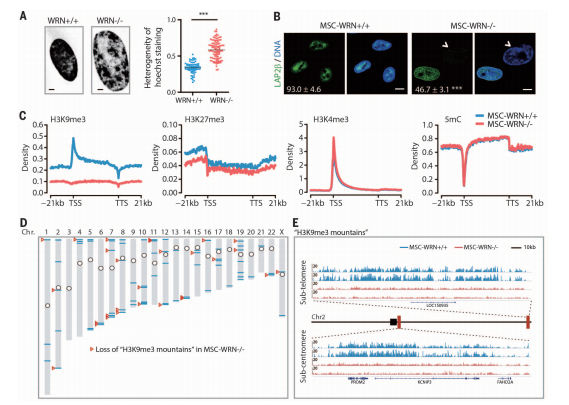
Epigenetic alteration has been considered as a hallmark of aging. MSCs-WRNŌłÆ/ŌłÆ showed a distinct nuclear Hoechst 33342 staining pattern with markedly enlarged nuclei and a high pixel-topixel coefficient of variation (CV) value, indicating possible changes in chromatin structure. Moreover, WRN-deficient MSCs exhibited accelerated diminishment of heterochromatinassociated inner nuclear membrane (INM) proteins LAP2b and LBR and reduced heterochromatin structure underneath the nuclear envelope, as indicated by immunostaining and electron microscopy. These results suggest a progressive disorganization of heterochromatin in WRN-deficient MSCs. Further investigation of heterochromatin reorganization at histone and DNA levels revealed marked down-regulation of the constitutive heterochromatin mark H3K9me3 (trimethylated histone H3 at lysine-9) in MSC-WRNŌłÆ/ŌłÆ. In contrast, H3K27me3 showed slight down-regulation, whereas H3K4me3, a mark for euchromatin fiber, exhibited comparable levels between WRN-deficient and wild-type MSCs. We did not observe obvious genome-wide alteration of 5-methylcytosine (5mC) in WRN-deficient MSCs (Fig. 2C). Bioinformatic analysis identified 73 H3K9me3-enriched ŌĆ£mountainsŌĆØ throughout the genome in MSCs-WRN+/+, which are characterized by >20 kb of consecutive peaks of H3K9me3 (Fig. 2D). Of these H3K9me3 mountains, 28 (38%) were lost in MSCs-WRNŌłÆ/ŌłÆ. Interestingly, 24 (86%) of these impaired H3K9me3 mountains resided in subtelomeric or subcentromeric regions.
RNA sequencing (RNA-seq) identified 1047 RefSeq genes that showed differential expression in MSCs-WRNŌłÆ/ŌłÆ (fig. S6, A and B, and table S2). The most obviously down-regulated genes were centromere-packaging proteins and components of the nuclear membrane (fig. S6, A to E, and table S3). These results indicate alterations in nuclear structure and epigenomic organization, potentially leading to a progressive loss of heterochromatin structure in MSCs as a consequence of WRN depletion. In agreement with previous reports describing WRN as a telomere-associated protein required for telomere maintenance, compromised telomerase activity and shorter telomere length were detected in MSC-WRNŌłÆ/ŌłÆ . In addition, chromatinimmunoprecipitationŌĆōquantitative polymerase chain reaction (ChIP-PCR) showed binding of WRN to the H3K9me3-enriched centromeric loci a-Satellite (a-Sat) and Satellite 2 (Sat2). Depletion of WRN resulted in an increase in centromeric g-H2AX signal and a loss of H3K9me3 from a-Sat and Sat2 loci accompanied by up-regulation of transcripts from these sequences.
Coimmunoprecipitation (Co-IP) analysis revealed WRN as part of a complex containing the major histone methyltransferase for H3K9me3ŌĆöSUV39H1, HP1a, and LAP2b, a nuclear envelope component that recruits heterochromatin via anchoring to HP1a. These observations
suggest a role for WRN, together with SUV39H1 and HP1a, in the stabilization of heterochromatin.
We next tested whether disorganization of heterochromatin could contribute to accelerated
cellular senescence. Knockdown of SUV39H1 or HP1a in wild-type MSCs led to a reduction of overall H3K9me3 and induction of cellular senescence, as assayed by Western blot, SA-b-gal staining, and p16 expression. On the contrary, overexpression of HP1a up-regulated H3K9me3 levels and repressed cellular senescence in WRN-deficient MSCs. To confirm these observations, we generated pluripotent ESCs-SUV39H1H324K lines harboring catalytically inactivated endogenous SUV39H1. Upon differentiation, MSCs-SUV39H1H324K displayed drastic nuclear structural and chromosomal changes, loss of INM proteins LAP2b and LBR, decreased levels of H3K9me3 and HP1a, up-regulation of centromeric repetitive sequence transcription, and coordinated transcriptional down-regulation of centromere-packaging components. MSCs-SUV39H1H324K recapitulated premature aging phenotypes observed in WRN-deficient MSCs, including retarded cell growth and accelerated cellular senescence determined by SA-b-gal staining High expression of SUV39H2, a germlinespecific histone methyltransferase, and/or other factors may functionally compensate for SUV39H1 deficiency in ESCs, where upon inactivation of the WRN-SUV39H1 axis, no discernible heterochromatin change was observed (figs. S10G and S8A). It should be noted that MSCs-SUV39H1H324K exhibit neither increased g-H2AX (P = 0.773) and phosphorylated ATM/ATR substrates (P = 0.279), nor telomere attrition. These results indicate that heterochromatin destabilization promotes premature aging in MSCs.
Finally, we asked whether heterochromatin disorganization could be a common hallmark for physiological human stem cell aging. For this purpose, we compared the levels of heterochromatin marks in primary dental pulp MSCs derived from six young (7- to 26-year-old) and six old (58- to 72- year-old) individuals. A marked down-regulation of WRN protein associated with a decreaseinH3K9me3,HP1a, SUV39H1, and LAP2b levels in MSCs derived from old individuals (Fig. 4E). Therefore, specific heterochromatin changes may underlie both pathological and physiological MSC aging. In summary, we have found that WRN protein, besides its role in DNA repair, functions to safeguard heterochromatin stability.
Our results reveal that the progressive heterochromatin disorganization observed in WRNdeficient MSCs underlies cellular aging, but more extensive studies are needed to examine its role during physiological aging. The methodologies and observations introduced here may be used and extended toward the systematic study of other age-associated molecular events with relevance to human aging and age-related disorders.
Source : http://www.sciencemag.org/
changes in heterochromatin architecture. We show that WRN associates with heterochromatin proteins SUV39H1 and HP1a and nuclear laminaŌĆōheterochromatin anchoring protein LAP2b. Targeted knock-in of catalytically inactive SUV39H1 in wild-type MSCs recapitulates accelerated cellular senescence, resembling WRN-deficient MSCs.
Moreover, decrease in WRN and heterochromatin marks are detected in MSCs from older individuals. Our observations uncover a role for WRN in maintaining heterochromatin stability and highlight heterochromatin disorganization as a potential determinant of human aging.
Werner syndrome (WS), also known as adult progeria, recapitulates certain aspects of human physiological aging. WS is caused by mutations in the WRN gene, resulting in loss of WRN expression or function. WRN protein plays roles in DNA replication, transcription, repair, and recombination, as well as telomere maintenance,
indicating that one of the major causes for WS pathogenesis relates to genomic instability. Epigenetic alterations have been associated with cellular aging in diverse model organisms. In humans, somatic cells derived from patients with premature aging syndromes are characterized by loss of heterochromatin marks. However, it is unclear whether epigenetic dysregulation is involved in WS pathogenesis.

Generation of patient-specific induced pluripotent stem cells (iPSCs) represents a promising avenue to model and study human aging and aging-associated disorders. WS-specific iPSC lines may constitute an ideal source for in vitro modeling of WS. However, we found that WS patient fibroblast lines deposited in different cell banks presented severe karyotypic abnormalities and secondary DNA mutations associated with advanced stages of WS pathology. To create an unbiased human WS cellular model, we sought to generate an isogenic WS embryonic stem cell
(ESC) line by knocking out exons 15 and 16 of the WRN gene encoding the conserved DNA helicase domain. After two rounds of homologous recombination using helper-dependent adenoviral vector (HDAdV), we successfully generated homozygous WRN-null ESC lines (ESCs-WRNŌłÆ/ŌłÆ ). ESCs-WRNŌłÆ/ŌłÆ expressed pluripotency markers, maintained normal karyotype, and could differentiate into all three germ layers.
ESCs-WRNŌłÆ/ŌłÆ lacked detectable WRN protein, as determined by Western blot using antibodies specific to the N or C terminus of WRN. No difference in cell cycle kinetics and cell growth rate between wild-type and WRN-null ESCs was observed.
WS patients are mainly characterized by premature aging pathologies associated with degeneration of mesodermal tissues, i.e., osteoporosis, atherosclerosis, and gray hair. We hypothesized that WS patients may suffer from an accelerated exhaustion of the mesenchymal stem cell (MSC) pool. This was tested by differentiating ESCsWRNŌłÆ/ŌłÆ intoMSCs. MSCs-WRNŌłÆ/ŌłÆ expressedMSCspecific cell surface markers CD73, CD90, CD105; lacked expression of MSC-irrelevant antigens, including CD45, CD34, and CD43 (fig. S3A); and could differentiate into osteoblasts, chondrocytes, and adipocytes. Upon serial passaging, WRN-deficient MSCs recapitulated major phenotypes of premature aging, including premature loss of proliferative potential, increased number of senescence-associatedŌĆōbgalactosidase (SA-b-gal)ŌĆōpositive cells, up-regulated expression of aging-associated genes p16Ink4a and p21Waf1, and activation of senescence-associated secretory phenotype (SASP) Moreover, when WRN-deficient MSCs expressing luciferase were transplanted into the muscle of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, they underwent an accelerated attrition compared to wild-type MSCs. These results demonstrated that the loss of WRN promotes premature senescence in MSCs.
WRN deficiency in MSCs resulted in elevated DNA damage response (DDR), indicated by increased nuclear foci for 53BP1, g-H2AX, and phosphorylated ATM/ATR substrates. Restoration ofWRN activity by lentivirus-mediated expression in MSCs-WRNŌłÆ/ŌłÆ resulted in partial alleviation of DDR and cellular senescence (fig. S4,
D and E). To investigate potential chromosomal abnormalities resulting from the loss of WRN protein,
we performed genome-wide copy number variation (CNV) analysis by deep sequencing. In the time frame examined, genomic integrity was minimally affected in MSCs-WRNŌłÆ/ŌłÆ.
Epigenetic alteration has been considered as a hallmark of aging. MSCs-WRNŌłÆ/ŌłÆ showed a distinct nuclear Hoechst 33342 staining pattern with markedly enlarged nuclei and a high pixel-topixel coefficient of variation (CV) value, indicating possible changes in chromatin structure. Moreover, WRN-deficient MSCs exhibited accelerated diminishment of heterochromatinassociated inner nuclear membrane (INM) proteins LAP2b and LBR and reduced heterochromatin structure underneath the nuclear envelope, as indicated by immunostaining and electron microscopy. These results suggest a progressive disorganization of heterochromatin in WRN-deficient MSCs. Further investigation of heterochromatin reorganization at histone and DNA levels revealed marked down-regulation of the constitutive heterochromatin mark H3K9me3 (trimethylated histone H3 at lysine-9) in MSC-WRNŌłÆ/ŌłÆ. In contrast, H3K27me3 showed slight down-regulation, whereas H3K4me3, a mark for euchromatin fiber, exhibited comparable levels between WRN-deficient and wild-type MSCs. We did not observe obvious genome-wide alteration of 5-methylcytosine (5mC) in WRN-deficient MSCs (Fig. 2C). Bioinformatic analysis identified 73 H3K9me3-enriched ŌĆ£mountainsŌĆØ throughout the genome in MSCs-WRN+/+, which are characterized by >20 kb of consecutive peaks of H3K9me3 (Fig. 2D). Of these H3K9me3 mountains, 28 (38%) were lost in MSCs-WRNŌłÆ/ŌłÆ. Interestingly, 24 (86%) of these impaired H3K9me3 mountains resided in subtelomeric or subcentromeric regions.
RNA sequencing (RNA-seq) identified 1047 RefSeq genes that showed differential expression in MSCs-WRNŌłÆ/ŌłÆ (fig. S6, A and B, and table S2). The most obviously down-regulated genes were centromere-packaging proteins and components of the nuclear membrane (fig. S6, A to E, and table S3). These results indicate alterations in nuclear structure and epigenomic organization, potentially leading to a progressive loss of heterochromatin structure in MSCs as a consequence of WRN depletion. In agreement with previous reports describing WRN as a telomere-associated protein required for telomere maintenance, compromised telomerase activity and shorter telomere length were detected in MSC-WRNŌłÆ/ŌłÆ . In addition, chromatinimmunoprecipitationŌĆōquantitative polymerase chain reaction (ChIP-PCR) showed binding of WRN to the H3K9me3-enriched centromeric loci a-Satellite (a-Sat) and Satellite 2 (Sat2). Depletion of WRN resulted in an increase in centromeric g-H2AX signal and a loss of H3K9me3 from a-Sat and Sat2 loci accompanied by up-regulation of transcripts from these sequences.
Coimmunoprecipitation (Co-IP) analysis revealed WRN as part of a complex containing the major histone methyltransferase for H3K9me3ŌĆöSUV39H1, HP1a, and LAP2b, a nuclear envelope component that recruits heterochromatin via anchoring to HP1a. These observations
suggest a role for WRN, together with SUV39H1 and HP1a, in the stabilization of heterochromatin.
We next tested whether disorganization of heterochromatin could contribute to accelerated
cellular senescence. Knockdown of SUV39H1 or HP1a in wild-type MSCs led to a reduction of overall H3K9me3 and induction of cellular senescence, as assayed by Western blot, SA-b-gal staining, and p16 expression. On the contrary, overexpression of HP1a up-regulated H3K9me3 levels and repressed cellular senescence in WRN-deficient MSCs. To confirm these observations, we generated pluripotent ESCs-SUV39H1H324K lines harboring catalytically inactivated endogenous SUV39H1. Upon differentiation, MSCs-SUV39H1H324K displayed drastic nuclear structural and chromosomal changes, loss of INM proteins LAP2b and LBR, decreased levels of H3K9me3 and HP1a, up-regulation of centromeric repetitive sequence transcription, and coordinated transcriptional down-regulation of centromere-packaging components. MSCs-SUV39H1H324K recapitulated premature aging phenotypes observed in WRN-deficient MSCs, including retarded cell growth and accelerated cellular senescence determined by SA-b-gal staining High expression of SUV39H2, a germlinespecific histone methyltransferase, and/or other factors may functionally compensate for SUV39H1 deficiency in ESCs, where upon inactivation of the WRN-SUV39H1 axis, no discernible heterochromatin change was observed (figs. S10G and S8A). It should be noted that MSCs-SUV39H1H324K exhibit neither increased g-H2AX (P = 0.773) and phosphorylated ATM/ATR substrates (P = 0.279), nor telomere attrition. These results indicate that heterochromatin destabilization promotes premature aging in MSCs.
Finally, we asked whether heterochromatin disorganization could be a common hallmark for physiological human stem cell aging. For this purpose, we compared the levels of heterochromatin marks in primary dental pulp MSCs derived from six young (7- to 26-year-old) and six old (58- to 72- year-old) individuals. A marked down-regulation of WRN protein associated with a decreaseinH3K9me3,HP1a, SUV39H1, and LAP2b levels in MSCs derived from old individuals (Fig. 4E). Therefore, specific heterochromatin changes may underlie both pathological and physiological MSC aging. In summary, we have found that WRN protein, besides its role in DNA repair, functions to safeguard heterochromatin stability.
Our results reveal that the progressive heterochromatin disorganization observed in WRNdeficient MSCs underlies cellular aging, but more extensive studies are needed to examine its role during physiological aging. The methodologies and observations introduced here may be used and extended toward the systematic study of other age-associated molecular events with relevance to human aging and age-related disorders.
Source : http://www.sciencemag.org/
Total 0
