The unusual pluripotency of neural crest cells is inherited from embryonic stem cells
By Stefan Hoppler and Grant N. Wheeler
What has made vertebrates so successful has been the evolution of their superior sensory organs, a more sophisticated organization of the brain, andŌĆöeventuallyŌĆötoothed powerful jaws, which gradually supported an ecological shift from passive filter feeding to a more active predatory lifestyle. Building this ŌĆ£new headŌĆØ in vertebrate embryos relies on an elite group of cells called the neural crest. Indeed, the English developmental biologist Peter Thorogood famously recounted that as a young scientist he was told by a senior professor (in an authoritative tone), ŌĆ£The only interesting thing about vertebrates is the neural crestŌĆØ. On page 1332 of this issue, Buitrago-Delgado et al. provide further support for this bold statement in demonstrating that neural crest cells uniquely retain pluripotent stem cell programming until later in development than the three classic germ layers.
The neural crest is a population of cells in vertebrate embryos with a remarkable ability to generate a variety of structures in the adult. The neural crest arises from within the so-called ectoderm germ layer, at the border between the future neural tissue and the future epidermis. However, unlike their neighbors on either side, neural crest cells undergo an epithelial-to-mesenchymal transition (EMT), during which they delaminate from the ordered tissue layer of the ectoderm and migrate as loose groups along defined paths to different and sometimes distant locations in the embryo, where they differentiate into a variety of cell types. Cell labeling and chimera experiments have been instrumental in uncovering these migration and differentiation patterns. Neural crest cells differentiate into cell types that generally arise from other germ layers such as the mesoderm, with the tissues they migrate through and those of their destination determining the cell types they become. Because this is particularly important for the formation of our ŌĆ£new head,ŌĆØ including the face, neck, and teeth, it is the neural crest that also allows us all to smile, or to frown.
Neural crest development in the vertebrate embryo is controlled by a largely conserved gene regulatory network, which has been described in detail. However, it had remained unclear where the remarkable differentiation ability of these cells came from. Prior to the study by Buitrago-Delgado et al., it seemed that neural crest cells were induced to selectively regain increased differentiation ability, unlike their cellular neighbors in the rest of the ectoderm. In this view, the neural crest could be described as an endogenous population of induced pluripotent stem cells.
Although many stem cell biologists liked this idea, it sat uncomfortably with traditional developmental biologists brought up on the paradigm of progressively restricted developmental potential, as illustrated by WaddingtonŌĆÖs developmental landscape.
Now, Buitrago-Delgado et al. provide insight into how neural crest cells retain far greater developmental potential than other ectoderm cells. They show that neural crest cells, rather than gaining developmental potential, retain pluripotency that they selectively inherit from the embryonic stem cells from which they are derived (see the figure, bottom panel). The authors noticed that neural crest potency factors are also expressed in pluripotent embryonic stem cells at an earlier stage of development. They show that these genes regulate pluripotency in these early embryonic stem cells, and that the experimentally extended expression of these factors can confer a delay in restricting developmental potential even to cells that would normally not give rise to neural crest. The maintenance of expression of these factors is what maintains neural crest differentiation potential.
Waddington can therefore rest in peace: Neural crest cells do not violate his paradigm of progressive restriction of developmental potential, they just manage to delay its onset for a little bit longer than their cellular neighbors. This remarkable differentiation ability ŌĆö at a stage of development when the three conventional germ layers have already formedŌĆöhad led to the neural crest being described as the ŌĆ£fourth germ layerŌĆØ. However, the new study shows that such a moniker is not required, because neural crest cells have now been shown to be little different from earlier embryonic cells; they are able to differentiate into cell types of all three conventional germ layers, including endoderm, albeit just a little bit later in development.
The work by Buitrago-Delgado et al. sheds further light on the evolution of the neural crest and therefore of vertebrates. Retaining pluripotency may not have been the crucial evolutionary step that defines the neural crest. Closely related nonvertebrate animals share with vertebrates the expression of components of the neural crest gene regulatory network in cells at the neural plate boundary, including what we now understand are regulators of pluripotency. Instead, vertebrate neural plate border cells may have evolved the ability to undergo EMT, delaminate from the ectoderm, and migrate to different parts of the embryo.
The Buitrago-Delgado et al. study raises questions about the identity of what we conventionally call the ectoderm. The most pressing issue is, however, how these cells at the neural plate border region selectively retain pluripotency while cells all around them become restricted in their developmental potential. Buitrago-Delgado et al. follow in the footsteps of pioneering studies that have used the amphibian as a model system to uncover fundamental aspects of vertebrate development, from basic processes in embryonic induction through uncovering the principle of somatic cell reprogramming. Like these earlier discoveries, now seen as pivotal in understanding human development and disease, this latest research has critically changed our perception of vertebrate evolution and development and demonstrates the importance of the neural crest.
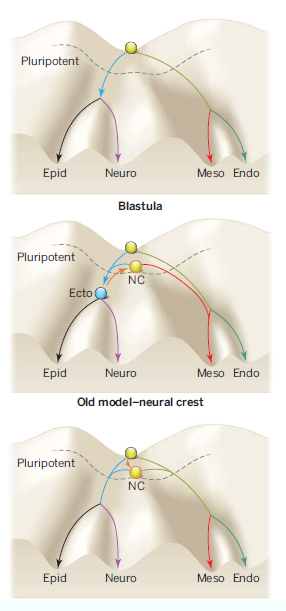
Pic. Developmental potential in embryonic development.
(Top) A pluripotent blastula or embryonic stem cell ŌĆ£rollsŌĆØ like a marble down one of four differentiation pathways: endoderm (Endo), mesoderm (Meso), neural ectoderm (Neuro), and non-neural ectoderm or prospective epidermis (Epid). The dashed line indicates where pluripotency is lost.
(Middle) Previous models implied that the differentiation ability of neural crest cells (NC, orange) was induced from an ectoderm germ layer (Ecto, blue), by ŌĆ£pushingŌĆØ the marble representing a neural crest cell uphill
toward increased differentiation potential back above the dashed line (orange arrow).
(Bottom) The new model by Buitrago-Delgado et al. shows that neural crest cells, unlike their cellular neighbors, remain pluripotent before they differentiate, even potentially into endodermal derivatives.
