Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours(2011)
BACKGROUND: Adoptive transfer of ex vivo expanded autologous Vg9Vd2 T cells may be of therapeutic benefit for cancer because of their potent direct cytotoxicity towards tumour cells, synergistic cytotoxicity when combined with aminobisphosphonates and enhancement of antibody-dependent cell-mediated cytotoxicity.
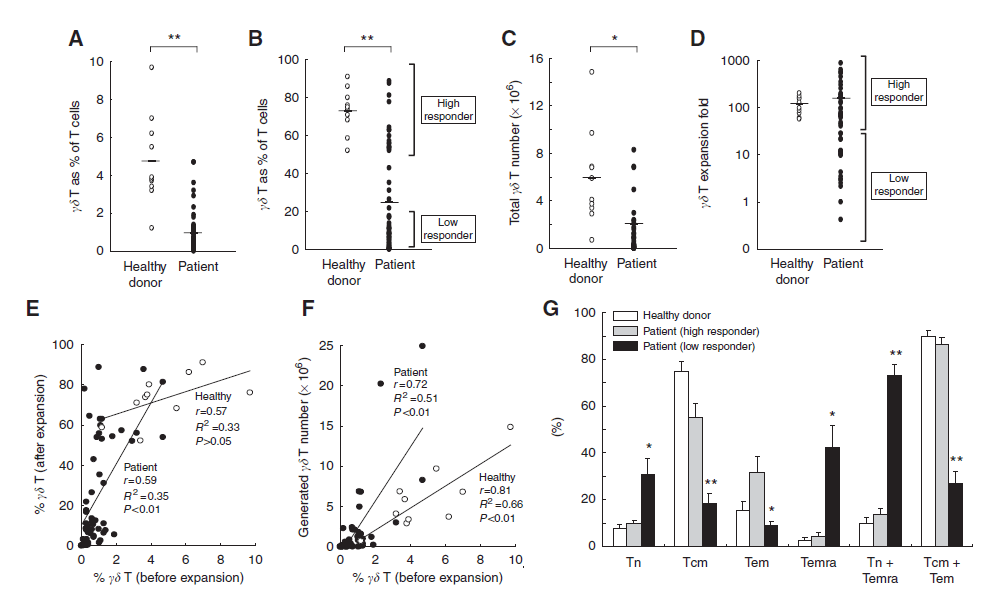
METHODS: To determine the feasibility and clinical safety of therapy with ex vivo expanded, activated Vg9Vd2 T cells in combination with zoledronate, we enrolled 18 subjects with advanced solid tumours into a phase I clinical study. Administered indium111-oxinelabelled Vg9Vd2 T cells were tracked in a cohort of patients.
RESULTS: Administered Vg9Vd2 T cells had an activated effector memory phenotype, expressed chemokine receptors predictive of homing to peripheral tissues and were cytotoxic in vitro against tumour targets. Adoptively transferred Vg9Vd2 T cells trafficked predominantly to the lungs, liver and spleen and, in some patients, to metastatic tumour sites outside these organs. No dose-limiting toxicity was observed, but most patients progressed on study therapy. However, three patients administered Vg9Vd2 T cells while continuing previously ineffective therapy had disease responses, suggesting an additive effect.
CONCLUSION: Therapy with aminobisphosphonate-activated Vg9Vd2 T cells is feasible and well tolerated, but therapeutic benefits appear only likely when used in combination with other therapies.
SOURCE :
British Journal of Cancer (2011) 105, 778ŌĆō786. doi:10.1038/bjc.2011.293
www.bjcancer.com
Published online 16 August 2011 & 2011 Cancer Research UK
Keywords: gamma delta T cells; Vg9Vd2 T cells; immunotherapy; clinical trial
