Aging, alopecia, and stem cells
— 2016-02-13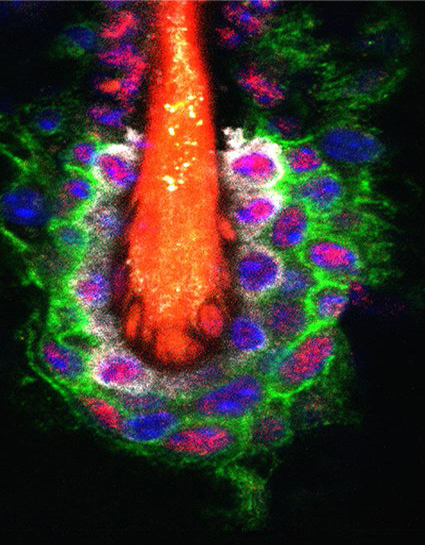
Hair follicle. The Foxc1 transcription factor (magenta) is present in stem cells (green) and their niche (white) during self renewal.
Some hope for restoring hair growth in people with alopecia.
Many tissues turn over during adult life, and declines in this process are associated with the progression of aging. Whether renewal is continual (as in the intestinal villi) or episodic (as in hair follicles), it is mainly attributed to somatic stem cells. One fundamental question is whether a decline of tissue renewal reflects the lifelong accumulation of external insults or the internal progression of a clock within stem cells?
Recently Research, ‘Foxc1 reinforces quiescence in self-renewing hair follicle stem cells(2016)’, shows insight into this phenomenon by examining hair follicle growth and aging.
Slowing down aging and moving toward regenerative medicine requires understanding fundamental principles of tissue renewal. If intrinsic changes in stem cells play a major role, the basis for their use to prevent aging weakens.
If environmental factors play a dominant role, rebuilding stem cell niches or modifying the global body environment will be important.
The hair follicle is an excellent model for studying regeneration because the follicle contains stem cells that can be activated cyclically, reflected in the growing (anagen) and resting (telogen) phases of the hair cycle. The longer the growth phase, the longer the hair.
Thus, the vitality of stem cells, reflected in the duration of the growth phase, is a measurable capacity. Because hair can show different phenotypes in different life stages and in different anatomical locations, it provides a highly accessible window to peek into the cellular and molecular basis of stem cell activity in growth and aging. Indeed, androgenetic alopecia (hair loss) is a common sign of aging.
- HFSCs = hair follicle stem cells
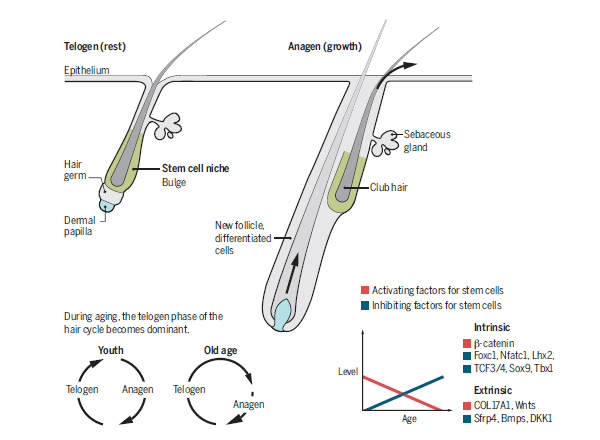
Hair growth and aging. Renewal capacity of the hair cycle decreases with aging. Intrinsic and extrinsic factors affect hair follicle stem cells. During aging, there is a shift from an activator-dominant to an inhibitor-dominant environment.
Yet the internal status of stem cells determines the response to these external factors. For example, deletion of DNA methyltransferase–1 (DNMT1) from the epidermis (in mice) decreases the probability of successful stem cell activation in every hair cycle, leading to progressive alopecia. This indicates that an intrinsic mechanism affecting the epigenetic landscape is involved in the response of HFSCs to external factors. As well, transcription factors such as LIM homeobox 2 (Lhx2), transcription factor 3/4 (TCF3/4), SRY-box 9 (Sox9), Tbox 1 (Tbx1), and nuclear factor of activated T cells, cytoplasmic 1 (Nfatc1) are critical to HFSC properties, and their absence results in accelerated entry into the hair growth cycle.
In androgenetic alopecia, hair fibers become shorter, thinner, and fewer as thick terminal scalp hairs are gradually replaced by fine vellus hairs. Yet in the early stages of this condition, HFSCs appear generally normal; a failure in their activation to form hair germ cells was the problem. Hair germ arises from activated HFSCs and migrate out of the bulge during early catagen to support the generation of a new hair. This suggests that the environment affects HFSCs. Such effects are not limited to the intrafollicle niche (the bulge); they also include the extrafollicular dermal environment and body hormone status. In aged mice, secreted factors in the intradermal adipose tissue that inhibit Wnt signaling [e.g., dickkopf wnt signaling pathway inhibitor 1 (DKK1) and secreted frizzled-related protein 4 (Sfrp4)] are present in a wider dermal region and persist for long amounts of time, thus blocking activation of hair growth and decreasing hair regeneration ability. The aging phenotype of the skin of an old mouse was partially rescued when transplanted into the dermal environment of a young mouse. Thus, the control of hair cycling during aging is likely affected by both intrinsic stem cell properties and extrinsic environmental factors. We need to learn more about how these different regulatory compartments interact to control HFSC activation.
The downstream effectors of dynamic histone modifications during hair cycling are considered to be good candidates for driving epigenetic change. Among these effectors is the transcription factor forkhead box C1 (Foxc1). Wang et al. show in mice that Foxc1 is expressed dynamically in HFSCs during hair cycling. It is absent during telogen (when stem cells are quiescent) but is expressed throughout the entire bulge when the next new hair cycle is initiated. Reducing Foxc1 expression in the basal hair follicle layer, where stem cells are located, resulted in a shortened telogen phase and loss of the old hair. Its absence in the suprabasal bulge caused a loss of club hairs (as new hair is formed, the hair fiber from the previous cycle—the club hair—is pushed upward in the follicle and eventually out). These results indicate that Foxc1 promotes HFSC quiescence and also maintains the niche structure. Wang et al. also show that genes that maintain HFSC quiescence are down-regulated in the HFSCs of mice lacking Foxc1. Chromatin immunoprecipitation (ChIP) sequencing and assay for transposase-accessible chromatin (ATAC) sequencing data revealed that several genes controlling HFSC quiescence contain Foxc1 binding sites in their promoter or enhancer regions. Remarkably, these include genes encoding Bmp and Nfatc1, factors that suppress HFSC activation. The findings push our understanding of hair cycle control from the morphogen level to an epigenetic level.
Extracellular matrix is a major component of the follicle stem cell niche. Among the matrix constituents, type XVII collagen is required to maintain both HFSCs and melanocyte stem cells. But how is the homeostasis of type XVII collagen regulated? Matsumura et al. show that during repetitive hair cycling, HFSCs accumulate DNA damage, which leads to proteolysis of type XVII collagen. They found that aging dorsal skin proceeds in a stepwise manner (in the mouse). Gene ontology analyses showed that genes involved in the DNA damage response are enriched in aged HFSCs, implying the accumulation of DNA damage during aging. Aged HFSCs produce ELA2/neutrophil elastase (ELANE) and other proteases that degrade COL17A1, the alpha chain constituent of type XVII collagen. Without COL17A1, HFSCs lose their self-renewal property and differentiate into an epidermal lineage. Thus, Matsumura et al. reveal an explicit mechanism for how aging related DNA damage in HFSCs loops back to alter the stem cell microenvironment.
Maintaining COL17A1 in the skin protected HFSCs against aging and reduced hair loss in mice. The study links DNA damage with changes of key extracellular matrix components in the stem cell niche, resulting in altered stem cell fate, depletion of stem cell numbers, reduction of organ size (miniature hairs), and reduced hair numbers.
This finding also raises the possibility of rejuvenating HFSCs, because the microenvironment is seemingly easier to modulate than the HFSCs themselves.
Tissue regenerative ability after wounding also declines during aging. In large wound–induced follicle neogenesis, new follicles form in addition to the regeneration of hair from the original follicles. However, this ability declines gradually with aging. Hair regeneration in aging persons is hormone-dependent and region-specific.
Androgens suppress hair growth in the scalp but enhance hair growth in beards and eyebrows. The findings of Wang et al. and Matsumura et al. should facilitate a multidimensional understanding of stem cell regulation and management during aging.
It may also provide some hope for restoring hair growth in people with alopecia.
1. L. Wang et al., Science 351, 613 (2016).
2. H. Matsumura et al., Science 351, aad4395 (2016).
3. Y. C. Hsu, E. Fuchs, Nat. Rev. Mol. Cell Biol. 13, 103 (2012).
4. E. Kandyba et al., Proc. Natl. Acad. Sci. U.S.A. 110, 1351 (2013).
5. C. M. Chuong et al., Physiology 27, 61 (2012).
6. V. Greco et al., Cell Stem Cell 4, 155 (2009).
7. V. A. Botchkarev, M. R. Gdula, A. N. Mardaryev, A. A. Sharov, M. Y. Fessing, J. Invest. Dermatol. 132, 2505 (2012).
8. W. H. Lien et al., Cell Stem Cell 9, 219 (2011).
9. J. Li et al., J. Invest. Dermatol. 132, 2681 (2012).
10. B. E. Keyes et al., Proc. Natl. Acad. Sci. U.S.A. 110, E4950 (2013).
11. L. A. Garza et al., J. Clin. Invest. 121, 613 (2011).
12. M. V. Plikus et al., Nature 451, 340 (2008).
13. C. C. Chen et al., J. Invest. Dermatol. 134, 2086 (2014).
14. S. Tanimura et al., Cell Stem Cell 8, 177(2011).
15. M. Ito et al., Nature 447, 316 (2007).
Source : Science Magazine. Feb, 2016




















Leave a reply
You must be logged in to post a comment.